Is Dipole Dipole Stronger Than Dispersion
HF boiling point 194 degrees Celsius has the strongest intermolecular forces. I know dipole-dipole and dipole-induced dipole forces are stronger than general dispersion forces but I noticed the dissociation energy is that what its called for all 3 types of attraction were -2 kJmol-1 in the lecture slides.

Intermolecular Forces Relative Magnitudes Of Forces The Types Of Bonding Forces Vary In Their Strength As Measured By Average Bond Energy Covalent Bonds Ppt Download
What is so special about hydrogen bonding.

. Why is hydrogen bonding stronger than dipole dipole. In large molecules which also have a large number of electrons which means that the electron cloud can be distorted to a greater degree London dispersion forces can be so. Accordingly how are dispersion forces similar to dipole dipole interactions.
The dipole-dipole interactions are due to interaction of partially positively charged a part of a molecule with the partially negatively charged part of the neighbouring molecule. The average strength of dispersion forces varies between1-10 kcalmol. Dipole-dipole forces occur between polar molecules as the force acts on.
The polarity differences in the bond and electronegativity differences affect the strength of dipole dipole interactions. Which noble gas has the weakest dispersion attractive force. These forces acts between the positive end of a polar molecule and the.
Ion-dipole forces are stronger than dipole interactions because the charge of any ion is much greater than the charge of a dipole. Dipole-dipole forces are stronger than London forces in small molecules. Part of the Van der Waals forces.
However London dispersion forces can be quite strong sometimes. Within intermolecular forces ion-dipole is the strongest followed by hydrogen bonding then dipole-dipole and then London dispersion. The strength of the ion-dipole force is proportionate to ion charge.
Dipole-dipole forces occur when there is an unequal sharing of electrons between two atoms. Because a hydrogen atom is so small these dipoles can also approach one another more closely than most other dipoles. Two hydrogen atoms and two lone non-bonding electron pairs.
Hydrogen bonding is a special type of dipole-dipole attraction between molecules not a covalent bond to a hydrogen atom. As a consequence they are not much a player. In larger molecules London forces tend to be stronger than dipole-dipole forces even stronger than hydrogen bonds.
The combination of large bond. But they are stronger than dipole-dipole and or dispersion forces. Dipole-dipole forces are stronger than the dispersion forces but weaker than ionic and covalent bonds.
Ionic bonding is stronger than any of the given intermolecular forces but is itself NOT an intermolecular force. Which compound has the strongest intermolecular forces. Negative end of the other molecule.
Which is stronger dipole-dipole or hydrogen bonding or London dispersion. Does H2S have dipole-dipole forces. In larger molecules London forces tend to be stronger than dipole-dipole forces even stronger than hydrogen bonds.
Ion-dipole hydrogen bonding dipole-dipole and Van der Waals forces. London dispersion forces occur when a positively charged nucleus of an atom attracts the electron cloud of another atom. Types of Intermolecular Forces- Dispersion DipoleDipole Hydrogen Bonding and Ion-Dipole.
Yes intramolecular forces are stronger than intermolecular force. Within intermolecular forces ion-dipole is the strongest followed by hydrogen bonding then dipole-dipole and then London dispersion. In this case the hydrogen bonding of water is stronger than the dispersion of H2Te.
These intermolecular ion-dipole forces are much weaker than covalent or ionic bonds. But when averaged over space and over orientations dipole forces cancel out one another. So if dipole dipole forces are being compared to intermolecular forces like London dispersion forces they would be stronger.
Hydrogen bonds vary from about 4 kJmol to 25 kJmol so they are still weaker than typical covalent bonds. Click to see full answer. Generally intramolecular forces are stronger than intermolecular forces.
Hydrogen bonds are stronger than dipole-dipole interactions because hydrogen bond is formed between highly electronegative atoms F O N and Which intermolecular force is weakest. Mwthane is not soluble in water because there are no poles that will participate in the dissolution process with water. They are weak because London dispersion forces are temporary forces 0-1 kcalmol.
Londons dispersion forces are weaker than dipole-dipole forces as they are because of momentarily dipoles. In order from strongest to weakest the intermolecular forces given in the answer choices are. Dispersion forces are weaker than dipole dipole interactions.
Dipole-Dipole vs Dispersion Force Strength. A weak intermolecular interaction arising from induced instantaneous dipoles in molecules. Because London dispersion forces are temporary theyre weaker than the permanent dipole-dipole attractions.
In general however dipole dipole interactions in small polar molecules are significantly stronger than London dispersion forces so the former predominate. Dipole-dipole forces have a weaker bond strength. Hydrogen bonds are are generally stronger than ordinary dipole-dipole and dispersion forces but weaker than true covalent and ionic bonds.
Dipole-dipole force is found in polar molecules which possess the positive. 36 Since dipole-dipole forces are stronger than dispersion forces NaCl will have higher boiling and melting points. But notice that here the amount of H2O is much greater than HCl and not 1 molecule of H2O is trying to break 1 molecule of HCl.
A typical energy of dipole-dipole interaction is 2 kJmol when molecules are stationary and 06 kJmol when rotating. The dispersion force is the weakest of all IMFs and the force. H2S H2Se and H2Te exhibit dipole-dipole intermolecular forces while H2O exhibits hydrogen bonding.
One on one permanent dipole-dipole force is much stronger than dispersion force. The positive and negative ends of different molecules. Why is a hydrogen bond considered stronger than other dipole-dipole intermolecular forces.
How do you know which intermolecular force is stronger. The molecular structure size and number of interactions affect the strength of dispersion forces. It is also highly soluble in water due to iondipole interaction that will prevail.
Hydrogen bonds are typically stronger than other dipole-dipole forces. To describe the intermolecular forces in liquids. Why are dipole-dipole forces the strongest.
In general however dipoledipole interactions in small polar molecules are significantly stronger than London dispersion forces so the former predominate. They are very important in the organization of biological molecules especially in.
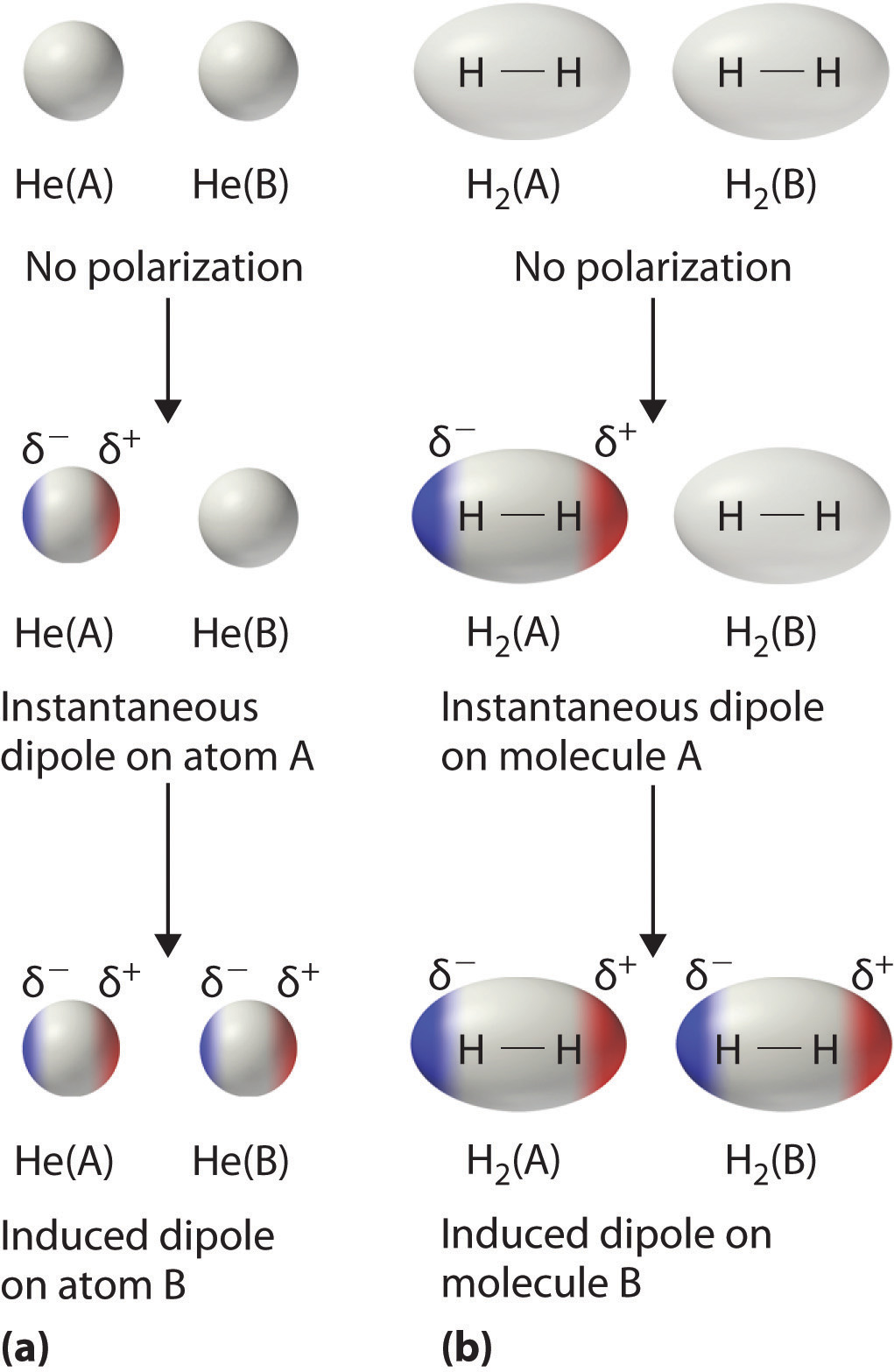
12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts
Difference Between Dipole Dipole And London Dispersion Forces Pediaa Com

Intramolecular Forces Vs Intermolecular Forces Intramolecular Forces Forces Within A Molecule Tend To Be Very Strong Hold The Atoms In Molecules Formula Ppt Download
No comments for "Is Dipole Dipole Stronger Than Dispersion"
Post a Comment